Key difference: These two words have the best bond, and there is no separation between them, which is nothing but “Atom and Molecule.” Both these terms are involved in chemical science. Atoms are the tiniest particle, whereas molecules are the mass of atoms. It has protons, electrons, and neutrons and consists of a nucleus in the middle of the atom.
The combining capacity of the element is known as Valency. Both atoms and molecules particles have valence electrons. All atoms are indivisible particles with different masses with variable physical and chemical properties. There is no free resistance in atoms, but molecules have it. Atoms are not chemically breakable, whereas molecules are chemically broken. Atoms are unstable alone, but molecules are stable alone.
The Atoms can’t be separated into sub-particles, but molecules can be separated into atoms. The standard atomic weight gives the mass of an atom, while molecular mass gives the molecule’s mass. In the article below, you can know the differences between atoms and molecules in detail.
Good Examples of the words “Atom and Molecule”
1. Atoms do not exist in a free state.
2. Molecules exist in a free state.
ATOM

Atom is the smallest particle.
Atom is the unit of particulate matter that denotes the chemical element, which consists of protons, electrons, and neutrons. Protons are positively charged, electrons are negatively charged, whereas neutrons are neutral. A combination of atoms is called a molecule. Generally, atoms have equal protons and electrons in number, giving them a neutral charge. Ions consist of anions and cations. Anions have extra and negatively charged electrons; Cations have deficient and positively charged electrons.
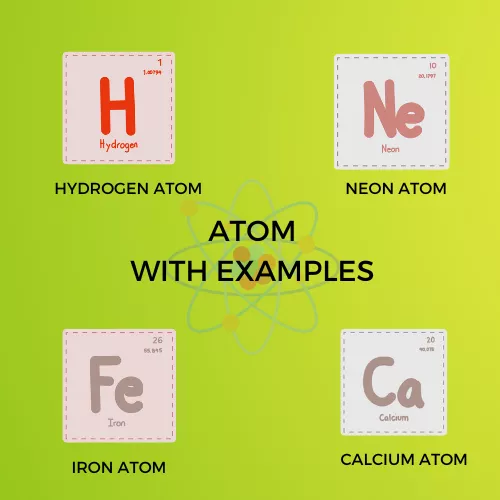
The Best example of an Atom is a “Hydrogen atom has no neutrons.”
How do We Spell the Word Atom?
The vocal representation of an Atom is “a·tm,” You can pronounce it this way. In the below, you can know by hearing the audio of the phrase atom.
Enunciation:
Syllabification is where we split the words into individual vowel sounds. A syllable should have at least one vowel in the word. For example, we will see the syllable of the word “Atom.”
- I wondered if the word “Atom” has two syllables.
- The split is “AT-OM.”
Using “Atom” in sentences:
- Electrons, Protons, and Neutrons are present in an atom’s nucleus.
- Science and technology developed the atomic bomb.
- The molecule is made up of more atoms.
- A particle that does not contain protons is not an atom.
- John Dalton is the father of atoms.
MOLECULE

The molecule is the compound element.
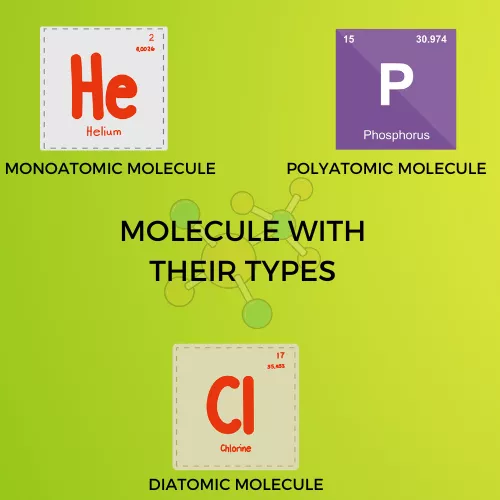
A molecule is made up of more atoms together. It has physical and chemical properties, where the chemical bonds hold the Molecule. There are 3 types of molecules: monoatomic, diatomic, and polyatomic. Then we can categorize the molecules as homonuclear is made up of only one element. An example of a homonuclear molecule is H2, O2, and a heteronuclear comprises two or more elements. An example of heteronuclear molecule is CH4, NO2, etc.,
The Best Example of the word Molecule is “H2 and CH4 are the lower molar mass molecules.”
How do We Spell the Word Molecule?
Spell / Pronunciation in which a word or particular language is spoken among people. The Oral representation of the phrase Molecule is “maw·li·kyool.” It would help if you practised it slowly for the outcome of a perfect spell. Below, you can know by hearing the audio of the word molecule.
Enunciation:
Syllabification is where we split the words into individual vowel sounds. A syllable should have at least one vowel in the word. For example, we will see the syllable of the word “Molecule.”
- Wondering if the word “Molecule” has three syllables in it.
- The split is “MOL-E-CULE.”
Using “Molecule” in sentences:
- Hydrogen and Chlorine elements are examples of molecules.
- Iron’s sulfide molecule is otherwise called fool’s gold.
- Proteins and Carbohydrates are the major classes of macromolecules.
- Molecules can be either homonuclear or heteronuclear.
- Water is a real-life example of a molecule.
Similarities between Atom & Molecule
The title reveals the difference between Atom & Molecule. But, like, you want to know also similar things about the above two words. So come, let’s see below.
- Both atoms and molecules are neutral in most cases.
- When two atoms bind to each other, they form a molecule. An example is Water, Chlorine, etc.,
Compare: ATOM & MOLECULE
| ATOM | MOLECULE | |
|---|---|---|
| Definition | Atom is the smallest unit of matter, with chemical characteristics and properties. | A molecule is a group of two or more atoms chemically bonds together. |
| Synonyms | Particle, Bit, Fraction, Trace, Shred, Scrap, Ounce, Grains, Fragment. | Atom, Corpuscle, Mote, Particle, Speck, Snippet, Patch, Lump, Chunk. |
| Reactivity & Stability | Reactivity is more, while stability is less. | Reactivity is less, while stability is more. |
| Energy Level | Energy is more | Energy is less |
| Shape | Spherical shape | Linear, triangular, and angular shapes. |
| Examples | If an atom is a loss, it is referred to as a cation. Whether the carbon atoms have a positive charge? The Atomic number of Oxygen is Eight. The atom is a basic building block in science. If an atom gains, it is referred to as an anion. | H20 is a chemically inert molecule. The most extended molecule is the polymer. Lipids come under macromolecules. Polar molecules are soluble in water. Is molecular formula available for all elements? |
Infographic Representation:
Resources & References:
Resources: Cambridge Dictionary (Atom & Molecule), Merriam-Webster (Atom & Molecule), Collins Dictionary (Atom & Molecule), Dictionary.com (Atom & Molecule) Reference: Dictionary.Cambridge.org[1], Merriam-Webster.com[2], CollinsDictionary.com[3], Dictionary.com[4].
External Links:
- https://dictionary.cambridge.org/dictionary/english/atom
- https://www.merriam-webster.com/dictionary/molecule
- https://www.collinsdictionary.com/dictionary/english/atom
- https://www.dictionary.com/browse/molecule
